Hello, without wanting to copy a current subject, I have bac -49 so birth.
What I can not understand, I search the internet so I need a simple example no link.
A refractory brick stores 880 joule per kilo, it is about 2.2 kg.
Let us suppose a surface of this material of 300cm 2 having only one point of exchange with the outside air. That is to say surrounded on the other faces of a R so enormous (style 1500 to cut short).
So I'm looking for the formula, but also the numerical calculation, to know how long, taking into account as an exchange point.
the 300cm 2 of exchange, the air which is therefore in contact with this surface is at 20 degrees.
So what is the number of joule of calories transferred in one second.
Hoping to be understandable in my question, which is not always my strong point.
a + claude
Heat transfer firebrick and air?
-
Christophe
- Moderator

- posts: 79364
- Registration: 10/02/03, 14:06
- Location: Greenhouse planet
- x 11060
Uh before getting into the heat exchange and heat diffusion equations (dedeleco will find them for you easily), what is the goal? Do thermal storage?
Water is much better than any solid material (usual, there may be better than water).
You can read this topic: https://www.econologie.com/forums/stockage-t ... t9567.html Or make a https://www.econologie.com/forums/search.php
Because storage has been seen many times across and across ...
Water is much better than any solid material (usual, there may be better than water).
You can read this topic: https://www.econologie.com/forums/stockage-t ... t9567.html Or make a https://www.econologie.com/forums/search.php
Because storage has been seen many times across and across ...
0 x
Do a image search or an text search - Netiquette of forum
Clasou writes, but he is very far from birth:
not precise enough in the units because it is 888J / Kg ° C, rather 840 on
http://fr.wikipedia.org/wiki/Diffusivit%C3%A9_thermique
(I can't do anything with the links, but I don't pretend to reinvent everything even at BAC + 40 !!!!, it's impossible, you need the measurements) and with the passage from 60 ° C to 20 ° C for 2,2Kg this represents a caloric reserve of 880x (60-20) x2,2 , 77,44 = XNUMXKJ
which will be dissipated on the surface of 300cm2 of contact with the outside air, very complex thermal convection problem, if we want to be exact, variable depending on where the brick is, inside or outside with what wind, mild or mistral at 100KM / h, depending on the orientation relative to this wind, which changes everything, as Clasou must have noted at 49 years old !!
Also by avoiding BAC + 6 to 40 links, to put it simply, the loss is that of the boundary layer of stationary air on the surface under fairly slow convection air currents or rapid mistral.
This insulating boundary layer has a variable thickness between 5 mm and 1mm approximately (with smoke we can see it and evaluate this quiet zone against the brick).
So if we take 5mm with air at 0,026W / mK with the link still
http://fr.wikipedia.org/wiki/Diffusivit%C3%A9_thermique
this gives 0,026 / (5mm / 1000mm) = 5,2W / m2K of losses or in reverse of R = 0,19m2K / W
or for 300cm2 = 0,03m2 a loss at the start with 60 ° C-20 ° C = 40 ° C
5,2x40x0,03 = 6,24 J / s
So if we forget the variation in the thickness of the boundary layer which increases with T approaching 20 ° C by reduction of convection, (important effect which slows down at the end) then the time to cool with an exponential T curve over time will be with a characteristic constant of:
77400 / 6,24 = 12400s = 3,44h
this is a simplistic assessment of the T in the center of the brick, but not the apparent one on the surface, which decreases much faster.
The weather changes greatly depending on the thickness of the boundary layer, can be half 2,5 mm and less if a little wind reducing to an hour. Convection is very complex, BAC + 10 and more !!
And it should be noted that we forget the diffusion of heat in the brick (BAC + 3 mini), which makes that on the surface the temperature decreases very quickly for a few minutes, but the interior remains hot thanks to the low thermal conductivity of the brick which slows down, and which I neglected, and which is roughly a penetration of 1mm for 1s, 10mm for 100s and 10cm for 10000s = 2,7 hours, but like the shape and dimensions of the brick have not been given, this cannot be calculated (complicated BAC + 5 and software) but probably as limiting as the external thermal contact, for this ordinary brick.
For much larger bricks, thickness of the meter, then this diffusion limits everything and for 3 to 6m, keeps the heat of the days and summer for the winter.
http://fr.wikipedia.org/wiki/Conduction_thermique
A refractory brick stores 880 joule per kilo, it is about 2.2 kg
not precise enough in the units because it is 888J / Kg ° C, rather 840 on
http://fr.wikipedia.org/wiki/Diffusivit%C3%A9_thermique
(I can't do anything with the links, but I don't pretend to reinvent everything even at BAC + 40 !!!!, it's impossible, you need the measurements) and with the passage from 60 ° C to 20 ° C for 2,2Kg this represents a caloric reserve of 880x (60-20) x2,2 , 77,44 = XNUMXKJ
which will be dissipated on the surface of 300cm2 of contact with the outside air, very complex thermal convection problem, if we want to be exact, variable depending on where the brick is, inside or outside with what wind, mild or mistral at 100KM / h, depending on the orientation relative to this wind, which changes everything, as Clasou must have noted at 49 years old !!
Also by avoiding BAC + 6 to 40 links, to put it simply, the loss is that of the boundary layer of stationary air on the surface under fairly slow convection air currents or rapid mistral.
This insulating boundary layer has a variable thickness between 5 mm and 1mm approximately (with smoke we can see it and evaluate this quiet zone against the brick).
So if we take 5mm with air at 0,026W / mK with the link still
http://fr.wikipedia.org/wiki/Diffusivit%C3%A9_thermique
this gives 0,026 / (5mm / 1000mm) = 5,2W / m2K of losses or in reverse of R = 0,19m2K / W
or for 300cm2 = 0,03m2 a loss at the start with 60 ° C-20 ° C = 40 ° C
5,2x40x0,03 = 6,24 J / s
So if we forget the variation in the thickness of the boundary layer which increases with T approaching 20 ° C by reduction of convection, (important effect which slows down at the end) then the time to cool with an exponential T curve over time will be with a characteristic constant of:
77400 / 6,24 = 12400s = 3,44h
this is a simplistic assessment of the T in the center of the brick, but not the apparent one on the surface, which decreases much faster.
The weather changes greatly depending on the thickness of the boundary layer, can be half 2,5 mm and less if a little wind reducing to an hour. Convection is very complex, BAC + 10 and more !!
And it should be noted that we forget the diffusion of heat in the brick (BAC + 3 mini), which makes that on the surface the temperature decreases very quickly for a few minutes, but the interior remains hot thanks to the low thermal conductivity of the brick which slows down, and which I neglected, and which is roughly a penetration of 1mm for 1s, 10mm for 100s and 10cm for 10000s = 2,7 hours, but like the shape and dimensions of the brick have not been given, this cannot be calculated (complicated BAC + 5 and software) but probably as limiting as the external thermal contact, for this ordinary brick.
For much larger bricks, thickness of the meter, then this diffusion limits everything and for 3 to 6m, keeps the heat of the days and summer for the winter.
http://fr.wikipedia.org/wiki/Conduction_thermique
0 x
Yes, well I'm not at 40 joule / kilo, especially since it is in memory.
Well, actually I'm going to make a rocket stove, so the goal is to try not to make it too small, but also not a monster that I will not be able to heat up.
The idea is not really to save, but above all it is comfort, style making a fire every two days.
But I just realized, proof that the nap is advice, that it is say the reverse of the way of calculating the losses of a wall.
therefore surface in m2 * temperature difference / lambda of the materials.
a + claude
Well, actually I'm going to make a rocket stove, so the goal is to try not to make it too small, but also not a monster that I will not be able to heat up.
The idea is not really to save, but above all it is comfort, style making a fire every two days.
But I just realized, proof that the nap is advice, that it is say the reverse of the way of calculating the losses of a wall.
therefore surface in m2 * temperature difference / lambda of the materials.
a + claude
0 x
I hate lambda because not clear and source of errors without the units !!
To be clear and avoid errors, you have to handle with the precise lambda W / mK units and not lambda alone, which means nothing without these very precise units !!
When writing the units we must end in J / s = W
and if lambda is the thermal conductivity in W / mK - not its inverse) it do not divide but multiply
loss = W / (mK) / m. m2 .K = W = J / s
loss in W = thermal conductivity (W / (mK) divided by the thickness of insulation in m, then multiply by the area m2, then multiply by the difference of T in K or ° C and there remains only the loss in J / s = W
so :
You shouldn't handle it by heart, but understand the principles with the units.
To be clear and avoid errors, you have to handle with the precise lambda W / mK units and not lambda alone, which means nothing without these very precise units !!
When writing the units we must end in J / s = W
and if lambda is the thermal conductivity in W / mK - not its inverse) it do not divide but multiply
loss = W / (mK) / m. m2 .K = W = J / s
loss in W = thermal conductivity (W / (mK) divided by the thickness of insulation in m, then multiply by the area m2, then multiply by the difference of T in K or ° C and there remains only the loss in J / s = W
so :
without the units does not allow us to understand and see the inconsistencies of what we are handling.surface in m2 * temperature difference / lambda of the materials.
You shouldn't handle it by heart, but understand the principles with the units.
0 x
No it is not a dewar, nor a bbc although it is more so far than that.
Anyway considering the shape of the rocket stove, a ton goes fast.
.
But my concern is let's say more the materials that I use, since some can store very high in temperature, but we have a price contrary to the idea of rocket which is more of the recovery.
So here is the assumption, but a sandbox -10 , it's not always easy.
, it's not always easy.
a + claude
Anyway considering the shape of the rocket stove, a ton goes fast.
.
But my concern is let's say more the materials that I use, since some can store very high in temperature, but we have a price contrary to the idea of rocket which is more of the recovery.
So here is the assumption, but a sandbox -10
a + claude
0 x
To put it simply, you can take almost anything as solid, cheap to store:
clay, brick, compacted earth, glass, sand, pebbles, limestone, granite, not necessarily refractory (if not in direct contact with the flames, just the smoke already cooled to 300 ° C), or even plaster or gypsum (less than 80 ° C, which will fall apart by heating too much), with approximately the value 0,88KJ / Kg ° K which gives per heated ton from 20 ° C to 120 ° C (difference 100 ° C) gives stored:
0,88x100x1000=88000KJ=88000/3600=24KWh/tonne de stockage
This stored the heat of combustion of 6Kg of dry wood at 4KWh / Kg.
This is low, because in 24 hours it only gives a power of 1KW of heating and in 8 hours only 3KW, on a working day.
So you have to heat the stones or bricks much more, to 220 ° C or even 320 ° C to have 2 to 3 times more reserve and 2 to 3 KW of heating over 24 hours and vouchers from 6KW to 9KW over 8 hours, for an absence of a working day.
With 2 tons we must have double that is 16h at 6 to 9KW of power, given back, so the house remains adequately heated.
A concrete block and cement house, isolated from the outside has 10 to 30 tons and therefore has an inertia of 1 to 2 days naturally, without mass stove, while in wood, much lighter, it has little inertia and the mass stove is useful.
That fixes the ideas, but one stores rather little per ton !!
and by Kg almost nothing.
Water stores 4,75 times more for the same weight, but refuses to exceed 60 to 80 ° C or with passage from 20 to 70 ° C (50 ° C variation) than 2,37 times more than bricks or stones heated to 120 ° C, and almost identical if heated to 220 ° C.
So water, with expensive tanks, is not interesting for storing this heat.
With paraffin or other phase change thing, we store more but hardly more than 5 times, for very expensive products (1000 to 2000 € per ton, or even more), compared to free clay taken underground .
see for an overview and not much better at less than 200 ° C without major long-term difficulties (see hydrated salts like gypsum, CaCl2):
360KJ / l = 0,1KWh / liter = 100KWh / m3 (m3 at 1,4 to 2,6 tonnes)
https://www.econologie.com/forums/post210883.html#210883
http://www.bine.info/hauptnavigation/pu ... el=1436%29
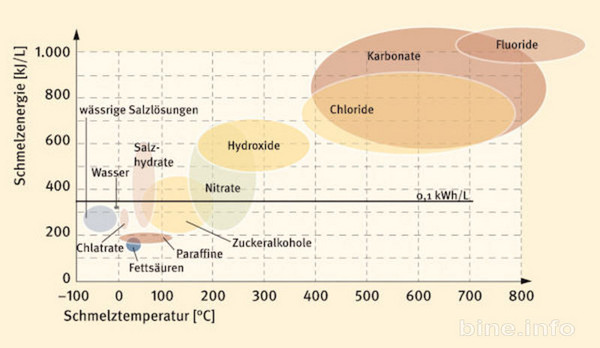
clay, brick, compacted earth, glass, sand, pebbles, limestone, granite, not necessarily refractory (if not in direct contact with the flames, just the smoke already cooled to 300 ° C), or even plaster or gypsum (less than 80 ° C, which will fall apart by heating too much), with approximately the value 0,88KJ / Kg ° K which gives per heated ton from 20 ° C to 120 ° C (difference 100 ° C) gives stored:
0,88x100x1000=88000KJ=88000/3600=24KWh/tonne de stockage
This stored the heat of combustion of 6Kg of dry wood at 4KWh / Kg.
This is low, because in 24 hours it only gives a power of 1KW of heating and in 8 hours only 3KW, on a working day.
So you have to heat the stones or bricks much more, to 220 ° C or even 320 ° C to have 2 to 3 times more reserve and 2 to 3 KW of heating over 24 hours and vouchers from 6KW to 9KW over 8 hours, for an absence of a working day.
With 2 tons we must have double that is 16h at 6 to 9KW of power, given back, so the house remains adequately heated.
A concrete block and cement house, isolated from the outside has 10 to 30 tons and therefore has an inertia of 1 to 2 days naturally, without mass stove, while in wood, much lighter, it has little inertia and the mass stove is useful.
That fixes the ideas, but one stores rather little per ton !!
and by Kg almost nothing.
Water stores 4,75 times more for the same weight, but refuses to exceed 60 to 80 ° C or with passage from 20 to 70 ° C (50 ° C variation) than 2,37 times more than bricks or stones heated to 120 ° C, and almost identical if heated to 220 ° C.
So water, with expensive tanks, is not interesting for storing this heat.
With paraffin or other phase change thing, we store more but hardly more than 5 times, for very expensive products (1000 to 2000 € per ton, or even more), compared to free clay taken underground .
see for an overview and not much better at less than 200 ° C without major long-term difficulties (see hydrated salts like gypsum, CaCl2):
360KJ / l = 0,1KWh / liter = 100KWh / m3 (m3 at 1,4 to 2,6 tonnes)
https://www.econologie.com/forums/post210883.html#210883
http://www.bine.info/hauptnavigation/pu ... el=1436%29

0 x
-
- Similar topics
- Replies
- views
- Last message
-
- 9 Replies
- 4498 views
-
Last message by phil53
View the latest post
07/04/12, 09:04A subject posted in the forum : Heating, insulation, ventilation, VMC, cooling ...
Back to "Heating, insulation, ventilation, VMC, cooling ..."
Who is online ?
Users browsing this forum : No registered users and 217 guests


