The purpose is not to use gravity, but to produce mechanical energy with the lowest possible temperature difference (in absolute terms, and according to the initial designer, some 10 degrees). In our case, if it could be run with a hot temperature of 50 ° C and a cold temperature of 15 ° C, the machine would be ideal. Indeed, we must try to work with available temperature without much transformation on earth.
Second, the initial design moves "volatile" and not "heat transfer" fluid and uses gravity in the same way.
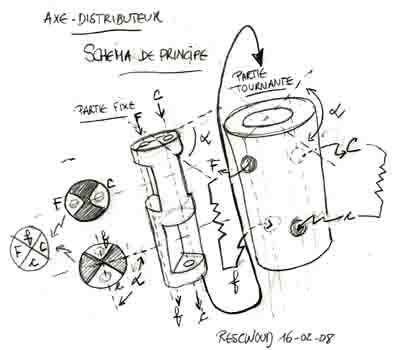
A Solar Engine: the Minto Wheel?
-
Pierre-Yves
- I understand econologic

- posts: 120
- Registration: 06/12/07, 17:13
- Location: Rennes-Quimper
rescwood wrote: The vaporization / condensation temperature differential, in my opinion, will mainly be related to the heat of vaporization: propane, 356 KJ / Kg, butane 362 KJ / Kg, DCM 341 KJ / Kg. This is where you have to go to get the mechanical energy recoverable by the system: the less thermal energy will be needed to raise the pressure remaining in temperature ranges close to the atmosphere (low boiling temperature). the better the efficiency of the transformation into mechanical energy.
this question of the fluid is fundamental. Butane is in the right range, but its explosive nature is unacceptable. I read somewhere that the exploitation of the thermal energy of the seas (thus with a low delta T between the surface water and that of the depths) had been realized (towards 1930) by using a mixture of water and ammonia, under vacuum. I would like to understand how it works: under vacuum, the boiling temperature is low, but the evaporation in a confined chamber increases the pressure which has the effect of also increasing the boiling temperature: the process must therefore 'Stop...
0 x
optimization, energy savings
http://www.avel-vor.fr
http://www.avel-vor.fr
- rescwood
- I understand econologic

- posts: 85
- Registration: 05/09/05, 14:30
- Location: Luxie (Southern Belgium)
Vincent:
Butane, like any substance whose vapors are flammable, is only flammable if it is in the presence of oxygen. Air Oxygen in our case: lower explosive limit 1.8% in air, upper explosive limit 8.8% in air. In no case, except poor sealing of the system, these conditions are met in the containers.
It is probably a heat pump, the system is different: the fluid evaporates on the "cold" side where the heat is pumped, this heat is then recovered during the condensation of the gas phase by a compressor.
In the Minto wheel, we do the opposite, we use the created pressure to create a displacement of fluid. To prevent a rise in pressure, cooling must be at least equal to the heat input into the system.
Butane is in the right range, but its explosive nature is crippling
Butane, like any substance whose vapors are flammable, is only flammable if it is in the presence of oxygen. Air Oxygen in our case: lower explosive limit 1.8% in air, upper explosive limit 8.8% in air. In no case, except poor sealing of the system, these conditions are met in the containers.
I read somewhere that the exploitation of the thermal energy of the seas (thus with a low delta T between the surface water and that of the depths) had been realized (towards 1930) by using a mixture of water and ammonia, under vacuum. I would like to understand how it works: under vacuum, the boiling temperature is low, but the evaporation in a confined chamber increases the pressure which has the effect of also increasing the boiling temperature: the process must therefore 'Stop...
It is probably a heat pump, the system is different: the fluid evaporates on the "cold" side where the heat is pumped, this heat is then recovered during the condensation of the gas phase by a compressor.
In the Minto wheel, we do the opposite, we use the created pressure to create a displacement of fluid. To prevent a rise in pressure, cooling must be at least equal to the heat input into the system.
0 x
- rescwood
- I understand econologic

- posts: 85
- Registration: 05/09/05, 14:30
- Location: Luxie (Southern Belgium)
Vincent,
the idea of moving a heavier fluid is interesting, but says heavier, says more energy to move it, so more pressure and therefore more heat. Clearly: with the same amount of heat used by the system, I will move the same mass, either a large volume of lighter fluid or a lower volume of heavier fluid ...
On the other hand, a heavier fluid would make it possible to obtain an interesting pair in smaller dimensions
the idea of moving a heavier fluid is interesting, but says heavier, says more energy to move it, so more pressure and therefore more heat. Clearly: with the same amount of heat used by the system, I will move the same mass, either a large volume of lighter fluid or a lower volume of heavier fluid ...
On the other hand, a heavier fluid would make it possible to obtain an interesting pair in smaller dimensions
0 x
-
Pierre-Yves
- I understand econologic

- posts: 120
- Registration: 06/12/07, 17:13
- Location: Rennes-Quimper
rescwood wrote:Butane, like any substance whose vapors are flammable, is only flammable if it is in the presence of oxygen ... In no case, except poor sealing of the system, these conditions are not met in the containers.
It's true, but you can never rule out leaks: I think of embedded systems with a lot of vibrations. If there was a non-toxic fluid, not dangerous for the environment and non-explosive, I'm interested! The new refrigerant liquids can be used, but it takes pressure from 4 to 14 bars, it is more difficult to implement.
I read somewhere that the exploitation of the thermal energy of the seas (thus with a low delta T between the surface water and that of the depths) had been realized (towards 1930) by using a mixture of water and ammonia, under vacuum. I would like to understand how it worked.
It is probably a heat pump, the system is different: the fluid evaporates on the "cold" side where the heat is pumped, this heat is then recovered during the condensation of the gas phase by a compressor.
In the Minto wheel, we do the opposite, we use the created pressure to create a displacement of fluid. To prevent a rise in pressure, cooling must be at least equal to the heat input into the system.
Not at all, it was not a CAP but, roughly speaking, the same principle as for the Minto wheel. The water-ammonia mixture (boiling point towards 0 °) was vaporized under partial vacuum with lukewarm water. The steam produced fed a turbine producing electricity, then was condensed by the cold source (water to 5 to 10 °).
Here is an excerpt from http://www.clubdesargonautes.org/ on the thermal energy of the seas:
"... In 1928, in Ougrée in Belgium, Claude validated the principle by producing electricity with a 60 kW thermal machine supplied with hot water at 33 ° C drawn from the cooling circuit of a blast furnace and “cold” water at 12 ° C pumped from the Meuse. Confirmed by the results of this experiment which allow him to show that the energy balance of the process is positive, Claude decides to demonstrate it in real conditions, no longer using fresh water but sea water brought by a cold water pipe of dimensions representative of those of a small industrial plant. "Claude then carried out an experiment with water of sea taken at the surface and taken at the bottom.
A delta T of 21 ° !!!! Can we imagine all the thermal sources that could be exploited?
It is the vacuum operation that I have trouble understanding, while it should not be too complicated yet!
0 x
- rescwood
- I understand econologic

- posts: 85
- Registration: 05/09/05, 14:30
- Location: Luxie (Southern Belgium)
It is the vacuum operation that I have trouble understanding, while it should not be too complicated yet!
I think I understood: we create a vacuum downstream of the turbine with a vacuum pump, which has the effect of lowering the boiling temperature of the sea water which vaporizes without being heated, instead to increase the upstream pressure by heating the sea water to vaporize it. Steam is "sucked" through the turbine instead of "pushing" it. Vaporized water is "hot" (surface) water, the vapor drawn in by the vacuum pump is then condensed by an exchanger supplied with "cold" (deep) water, thus increasing the depression created by the vacuum pump . Apparently, the energy consumed by the vacuum pump and the seawater feed pumps is less than that which would be required to vaporize the same water at atmospheric pressure.
Apparently Ammonia is used in another circuit, closed, to make the ice, circuit whose condenser and evaporator are also fed with cold water and hot (there are no small profits!) And whose compressor is powered by the current produced by the turbine as the vacuum pump and the pumps of the seawater circuit.
So, I'm good?
0 x
-
Pierre-Yves
- I understand econologic

- posts: 120
- Registration: 06/12/07, 17:13
- Location: Rennes-Quimper
rescwood wrote:I think I understood: we create a vacuum downstream of the turbine with a vacuum pump, which has the effect of lowering the boiling temperature of the sea water which vaporizes without being heated, instead to increase the upstream pressure by heating the sea water to vaporize it. We "suck" the steam through the turbine instead of "pushing" it. Vaporized water is "hot" (surface) water, the vapor produced is condensed by an exchanger supplied with "cold" (deep) water, thus increasing the depression created by the vacuum pump. Apparently, the energy consumed by the vacuum pump and the seawater feed pumps is less than that which would be required to vaporize the same water at atmospheric pressure.
So, I'm good?
that's not it yet! I try to understand, me too and I think at the same time as I write!
Surface seawater is the hot source and is used to vaporize the water-ammonia mixture. It is this steam that drives the turbine and is condensed by the cold source (seawater depths, at a temperature roughly constant 5 °). If it worked at atmospheric pressure (with an ideal fluid that would evaporate to 10-15 °), it would probably be enough for a simple circulator and / or a check valve for the system to work by itself.
What I do not understand yet is the operation under partial vacuum, which is necessary to lower the boiling point of the water-ammonia mixture. Indeed, if this mixture vaporizes, as the enclosure is confined, the pressure will increase and boiling will stop. It is necessary to evacuate the steam to maintain the depression, then to use this steam to make run the turbine ...
Is the depression created by condensation AFTER the turbine enough to maintain the cycle? Is the vacuum pump still running? Is there a chemist in the room?
0 x
-
- Similar topics
- Replies
- views
- Last message
-
- 3 Replies
- 1795 views
-
Last message by Remundo
View the latest post
14/01/23, 22:21A subject posted in the forum : Solar thermal: solar collectors CESI, heating, hot water, stoves and solar cookers
-
- 62 Replies
- 59230 views
-
Last message by Christophe
View the latest post
09/06/17, 13:53A subject posted in the forum : Solar thermal: solar collectors CESI, heating, hot water, stoves and solar cookers
Back to "Solar thermal: solar collectors CESI, heating, hot water, stoves and solar cookers"
Who is online ?
Users browsing this forum : No registered users and 86 guests