The properties of water: isotopes and molecular structure.
The properties of water 1: general
The properties of water 2: physical and chemical properties
Isotopic composition of water
Water is a mixture of various combinations of oxygen and hydrogen isotopes differing from each other in the number of neutrons associated with the protons in the nucleus.
1H,2 H (deuterium)3H (Tritium)
16O, 17O,18O.
The isotopic ratios are:
For hydrogen:
2H/1H = 1 / 6900
3H/1H = 1 / 10 18
Tritium is an unstable element, its time (half-life) is 12,5 years.
For oxygen:
18O/16O = 1 / 500
17O/16O = 1 / 2500
4 the main molecular species and their frequency is as follows:
1H216O = 99,7%
1H2 18 O = 0,2%
1H217O = 0,04%
1HD16O = 0,03%
D216O = very low
Different isotopes induce differences in the physical properties of molecules, in particular their density, but the chemical properties remain the same.
Heavy water D2O exists in its natural state but in very
low. To have an appreciable quantity of it, it is necessary to master the techniques of isotope separation: this was one of the fundamental challenges during the last world war to prepare the atomic weapon.
The isotopic composition of the chemical components of water is used in the estimation of thermodynamic parameters such as temperature;
The report 18 O/16O ice from the polar caps and water from fossil groundwater provides information on the climate of the past.
Evaporation of ocean water takes place with isotopic fractionation: the light isotope of oxygen evaporates in preference to the heavy isotope. The oceans are richer in heavy isotopes than water from clouds and precipitation.
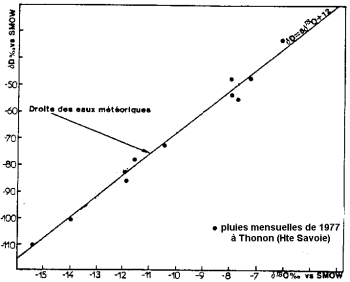
Stable isotope content of precipitation (after Blavoux and Letolle, 1995).
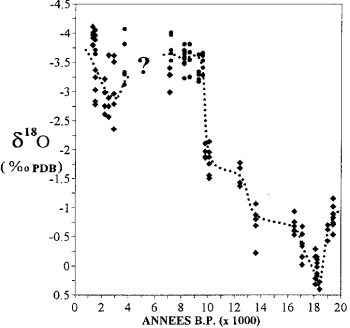
Variation in the oxygen isotope content in corals in Mayotte (after Casanova et al., 1994).
Structure of the molecule
Hydrogen and oxygen atoms pool their electrons to form a complete layer like that of neon. Indeed, the oxygen atom lacks 2 electrons to complete its electronic shell, it is the 2 hydrogen atoms that provide it. The H2O molecule formed is stable.
Oxygen: 8 8 protons + neutrons
Hydrogen: 2 (2 * (1 1 neutron proton +))
Total: protons 10 10 balancing loads of electrons.
The hydrogen nuclei have one side of that of oxygen to form a characteristic “Mickey's head” (the hydrogen being the ears).
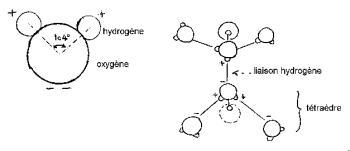
The HOH angle is 104,474 ° (characteristic of tetrahedral geometry). The distance between oxygen and hydrogen atom is close to 1 A ° (0,95718 A °) in steam. The effective diameter of the molecule is of the order of 2,82 A °.
Electric charges are unevenly distributed in this small molecule. Electrons are more strongly attracted to the oxygen atom than to the hydrogen atom. There are 2 centers of positive charges near the hydrogen nuclei and 2 centers of negative charges near the oxygen nucleus. This imbalance in the distribution of charges, combined with the nonlinear geometry of the water molecule, manifests itself in the existence of a strong electric dipole moment. The water molecule is polar; it behaves like an electric dipole which can thus bind with other polar molecules. Indeed, water molecules can be inserted between the constituent ions of a crystal by directing their part of opposite electric charge towards them. The attraction of crystalline ions is greatly weakened and the cohesion of the crystal is reduced, which facilitates its dissolution. The polar properties of the water molecule explain the technique of microwave heating. Indeed, a polarized molecule orients itself in relation to an electric field; if this varies, the molecule follows the change in orientation. From a certain frequency, a few GHz for water, the movements of molecules produce heat by friction. Household ovens typically operate at a frequency of 2,45 GHz, which is UHF.
The 3 nuclei of the molecule are not stationary, they move relative to each other, the molecule vibrates and twists. In liquid water, molecules tend to associate: Mickey's heads bond ear to chin by hydrogen bonding. Indeed, out of the 8 peripheral electrons of oxygen, only 4 are involved in covalent bonds with hydrogen atoms. The remaining 4 electrons are grouped into 2 pairs called free electron doublets. Each of these negative electrically charged doublets can form an electrostatic bond with a positively charged hydrogen atom from a nearby water molecule. The hydrogen bond, which is stable at room temperature, is nevertheless fragile compared to the covalent bond. In the water molecule, the geometry formed by the direction of the 2 covalent bonds and the 2 free electronic doublets is close to that of a tetrahedron whose center is occupied by the oxygen nuclei.
However, the large-scale structure of the water molecule is still imperfectly known. The X-ray and neutron diffraction spectra provide 2 main values: a signal corresponding to 1 A °, distance between the hydrogen and oxygen nuclei, and a value of 2,84 to 4 A ° varying according to the temperature and corresponding to the distance between 2 oxygen nuclei. RX diffractometry also makes it possible to know the average number of molecules per unit volume of liquid located at a distance R from a given molecule. A water molecule has an average of 4,4 neighbors, which suggests a tetrahedral mesh. In addition to molecules linked by hydrogen bonds, there are other unbound molecules, which may explain why the number of neighboring molecules is slightly greater than 4, and not 4 exactly as a strict tetrahedric crystallized state would impose. The crystal lattice of molecules linked by hydrogen bonds would form cavities where unbound molecules would be housed. Another hypothesis is based on the distortion of hydrogen bonds. The latter, originally linear, that is to say with the O – HO atoms aligned, could twist to varying degrees and allow molecules that are more distant than the close neighbors to approach the central molecule.
Theoretical models have recently been developed using powerful computers. They indicate that about 80% of water molecules are involved in 3 or 4 hydrogen bonds; on the other hand, they exclude the presence of unbound molecules. Computer modeling suggests that as the water cools, the networks of molecules increasingly resemble hexagons similar to those in ice.
The solid state corresponds to a stricter crystalline arrangement. At ordinary pressure, ice has a hexagonal structure. At low temperature (below -80 ° C), it can take a cubic structure. Electric charges can move in the crystal lattice and produce ionic type crystal defects: hydrated proton H3O + and hydroxyl ion OH-. The crystal lattice of ice does not correspond to the most compact possible stacking of molecules. Upon fusion, the defects collapse because the hydrogen bonds break and the molecules come closer together: the density increases to a maximum at 4 ° C. Subsequently, in liquid water, the increase in temperature pushes the molecules apart and the density decreases.
Details, references and bibliography:
Blavoux B. and Letolle R. (1995) - Contribution of isotope techniques to the knowledge of groundwater. Geochronics, 54, p. 12-15.
Caro P. (1990) - The physical and chemical properties of water. The great book of water, La Villette, p. 183-194.
Eagland D. (1990) - The structure of water. La Recherche, 221, p. 548-552.
Maidment DR (1992) - Handbook of hydrogeology. Mc Graw Hill.
Casanova J., Colonna M. and Djerroud K. (1994) - Geoprospective - paleoclimatology. Rapp. scient. BRGM, p. 76-79.

